Stem cells. Cells with unique properties to renew damaged tissues or organ functions. Cells that can be found in almost any tissue, but only in a reduced number.
Cell2B is working on the development of advanced cellular therapies. Together with the development of new proprietary techniques and protocols, they aim to increase the number and effectiveness of stem cells. The so important cells for tissue and organ regeneration.
Cell2B, a biotechnology company, has one goal: to deliver a new set of cell therapies to people who tackle immune and inflammatory malignancies.
The company with a visionary thinking was officially incorporated in 2011. However, their work started way before. Since 2005, 4 PhD students of Bioengineering have worked hard on the development of a product that could solve these problems. One evening, they all met for a dinner in a cozy restaurant in Boston. And Boston is where the idea of Cell2B was born. David Braga Malta, recalls the beginnings of Cell2B:
“We talked about putting everything together and merging into a company. We all agreed that it was the right move.”
We did a pilot study with these patients, to try and see if the technology was efficacious or not. And it was! – David Braga Malta
With the support from the Lisbon University and mentoring from MIT, Cell2B technologies got ready for the first pilot study. From 2007 to 2009 they treated patients in Portugal with Graft-versus-Host Disease. Also known as GVHD, it is a major complication, when white blood cells in the donated bone marrow attack your own body. Patients who suffer from GVHD have no alternative. There is a 95% mortality rate for the most severe cases.
However, this pilot study was conducted on only 6 patients, which in the pharmaceutical industry doesn’t show powerful statistics. To have a more robust product in terms of manufacturing and clinical efficacy in treating the disease, the Cell2B team has evolved their manufacturing process. GVHD, a fully developed product, will be tested in a real environment to show whether it works or not. At the moment, Cell2B is preparing for larger clinical studies, to show with statistical power if their product can help other people in need of this treatment. Their first 6 patients are doing very well. David Braga Malta shares his excitement: “The results are better than what they were supposed to be. We got very good results, what is really promising.”
The clinical development to reach the market is going to take 6 years. With the kick-off of the clinical trial planned for next year, Cell2B believes in one thing:
“We believe that by 2020 Immunesafe® will give enough proofs in the clinics to be granted a marketing authorization by the European Agency.”
It can seem a small goal for next year, but this clinical study tackles a lot of different parts. From manufacturing to clinical operations or funding – “The majority of our funding goes to this clinical study.”
Funding for life sciences in general is tough.
And funding is one of the biggest obstacles for Cell2B. Even though they raised already $2 million, for a biotech company like Cell2B, it is not enough. David Braga Malta tells us a bit about the struggles with funding:
“Funding for life sciences in general is though. If your startup is outside the US, more precisely Boston, it can truly be hard. Financing is always a challenge. We were able to fund the company with local business angels from Portugal. Currently, we are in the middle of raising a Series A with international VCs and some national players.”
Malta believes that one of the reasons why biotech companies struggle to get funding in Portugal, is that there is no specialized venture fund for the life sciences.
With 2 products in development, Cell2B is aiming to shift the paradigm in medicine: “In medicine, we treat the symptoms and not the cause of the disease. In some cases we manage to get better with treating only the symptoms. But sometimes our bodies are not able to recover naturally.”
We hope to shift the paradigm from symptom treatment to cause curing.
And that is where Cell2B comes in. Immunesafe® and Cordsafe®, the products of Cell2B, are able to target the cause, completely reverse it and cure the diseases the body is tackling. The co-founder of Cell2B shares their vision: “We hope to shift the paradigm from symptom treatment to cause curing.”
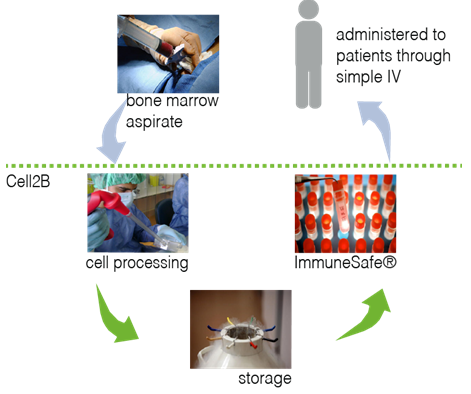
Immunesafe® is not only targeting acute steroid-refractory GVHD. Inflammatory bowel disease, acute liver failure or rejection of solid transplants are a few of the diseases which can also be treated by Immunesafe®. All thanks to its ability to modulate the immune and inflammatory responses in our bodies. Immunesafe® is an animal-free cell product and the first product under clinical evaluation for several indications. This product is establishing new standards in the Regenerative Medicine field.
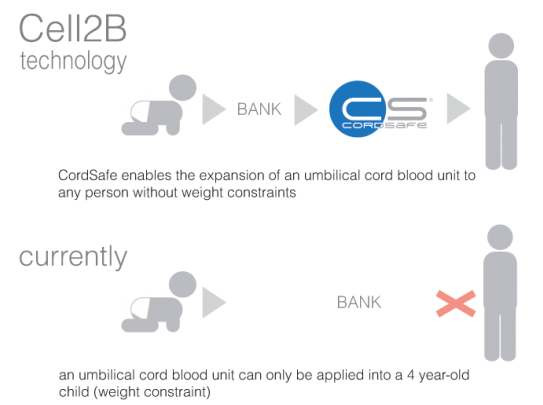
Cordsafe® is a product which will change the way we use cord blood units. Whilst the pharmaceutical industry is using cord blood units only for patients with up to 40 kg, Cordsafe® enables using cord blood units in adult patients, just like me or you.
The awards for Cell2B are not just the product of hard work, but also a moral booster. Working on long projects such as Cell2B, can be hard on the team’s motivation:
“One very important part of the motivation of the team, is the dream. Our ultimate goal is very ambitious, but at the same time very real. Everybody who is part of Cell2B has the same end goal and is really motivated to do their best to get there. Winning awards is also a very good moral boost and it helps to keep everybody in the company motivated and on the same page.” – David Braga Malta
Cell2B is turning the biotech industry up-side-down. “Everyone sees that, if we will be able to achieve what we propose, this will be a disruptive change in the field” – David Braga Malta